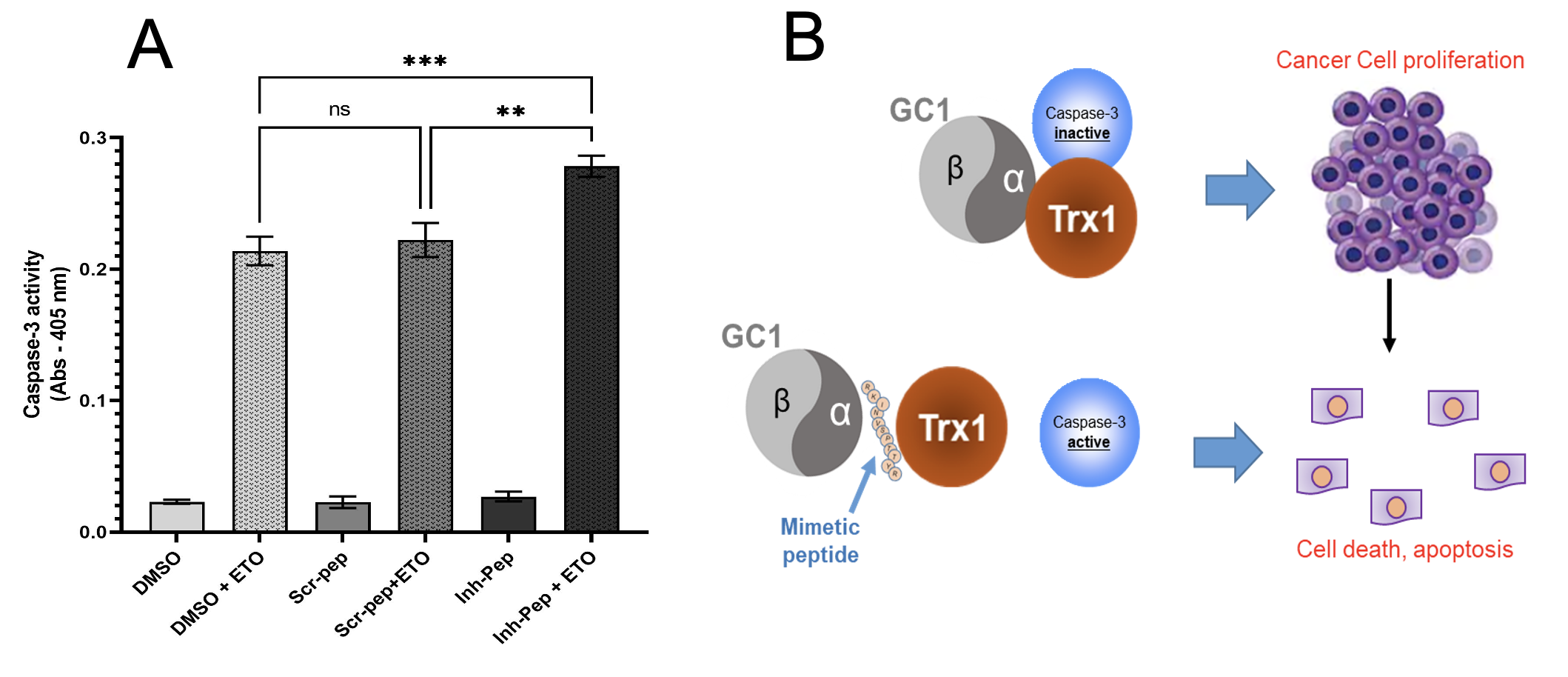
 Inhibitory peptide increases caspase3 activity (A) by blocking GC1/Trx1 complex thereby limiting cell proliferation by inducing cell death (B).
Inhibitory peptide increases caspase3 activity (A) by blocking GC1/Trx1 complex thereby limiting cell proliferation by inducing cell death (B).
Invention Summary:
Soluble guanylyl cyclase (GC1) and thioredoxin 1 (Trx1), when physically interacting and attaching to each other, they acquire the ability to induce changes in other target proteins such as caspase‐3, modifying their properties. The complex GC1/Trx1 modifies caspase‐3 and makes it inactive with an outcome where cell death is inhibited, and proliferation of tumor cells proceeds.
Rutgers researchers have developed a novel “mimetic peptide”, 10-12 amino acids long, that blocks the ability of GC1 and Trx1 to form a complex. The ability to modify and inactivate caspase‐3 is lost as the complex is disrupted. When this peptide is added to leukemic Jurkat T cells, caspase‐3 activity is restored, and allows a programmed cell death, blocking cell proliferation. A mimetic peptide that blocks the attachment between GC1 and Trx1 and consequently blocks the modification of targets highlights the novelty of this invention.
Advantages:
• The peptide is accessible to tumors and blocks tumor proliferation.
• The peptide slows down tumor development.
• The peptide can be used as a tool to study subsequent target modification in other tissues and assess the pathophysiological relevance.
Market Applications:
• Breast, endometrial and cervical cancers
• Prostate cancer
Intellectual Property & Development Status: Provisional patent application filed, patent pending. Available for licensing and/or research collaboration. For any business development and other collaborative partnerships contact marketingbd@research.rutgers.edu.
Publications: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC10135718/