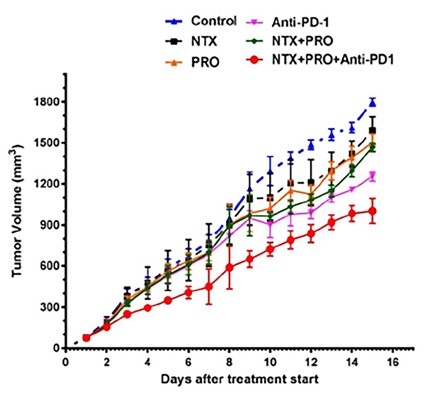
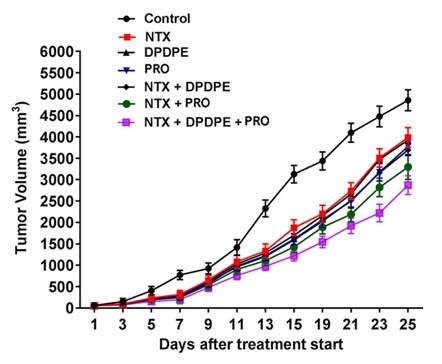
Figure 1. (Left) Combined, synergistic treatment with naltrexone (NTX), propranolol (PRO), and Anti-PD1 antibody shows decrease in tumor size, by volume. (Right) Combined treatment of NTX, DPDPE, and PRO similarly decreases tumor size compared to controls.
Invention Summary:
One of the major cancer therapy approaches that is being heavily investigated is immunotherapy. Immunotherapy involves modulating the cancer patient’s own immune system to identify tumors and cancer cells as foreign to their body. Currently, the major pathways being studied involve regulating the function of T-lymphocytes via CTLA-4 and PD-1 as these cells’ primary function is to distinguish host cells from foreign cells. However, immunotherapies involving drugs that influence other cellular pathways are limited in the market.
Rutgers researchers have identified that DPDPE, naltrexone, and propranolol can be combined with PD-1-based immunotherapies to improve cancer treatments. The researchers show that cancer cell lines from breast and colon cancers treated with this novel cocktail decreases cells’ proliferation rate and increases their apoptotic rate. The addition of the three drugs increases immune responses, synergizing with the effect of the traditional, PD-1 antibody-based treatment. In addition, xenograft models in rats validate the cell lines results with decreased tumor sizes in rats treated with the combination of drugs.
Advantages:
- DPDPE, naltrexone, and propranolol are drugs that are approved by FDA
- Increased efficacy of already-used cancer treatment
- General treatment for boosting innate immune system’s response
Market Applications:
- Adjuvant for treatment of broad cancer types
- Immunomodulation for improving response to treatment of other diseases
Intellectual Property & Development Status:
PCT application filed, patent pending. Available for licensing and/or research collaboration. Please contact marketingbd@research.rutgers.edu.
Publications & Clinical Trials:
Phase 1 Clinical Trial (Recruiting): NCT05968690
Murugan S, Rousseau B, Sarkar DK. Beta 2 Adrenergic Receptor Antagonist Propranolol and Opioidergic Receptor Antagonist Naltrexone Produce Synergistic Effects on Breast Cancer Growth Prevention by Acting on Cancer Cells and Immune Environment in a Preclinical Model of Breast Cancer. Cancers (Basel). 2021 Sep 28;13(19):4858.
Rousseau B, Murugan S, Palagani A, Sarkar DK (2022). Beta 2 adrenergic receptor and mu opioid receptor interact to potentiate the aggressiveness of human breast cancer cell by activating the glycogen synthase kinase 3 signaling. Breast Cancer Res. 2022 May 14;24(1):33.