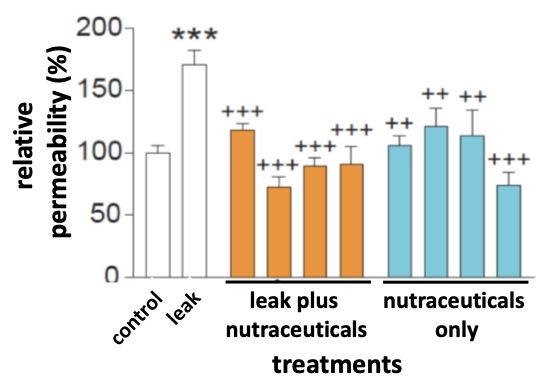
Image Legend: Control intestinal organoids and organoids conditioned to have a leak, or more permeable barrier, were treated with nutraceuticals which clearly prevented increased permeability in the “leaky” organoids. Similar findings were observed in cell culture and in vivo
Invention Summary:
More than 22 million Americans suffer from chronic autoimmune and inflammatory diseases that are linked to a “leaky” gut, i.e., easily permeable for inappropriate entry by bacteria and toxins into the blood. Certain beneficial bacteria living in a healthy human gut (probiotics) protect gut wall integrity by producing protective substances via host-microbial interaction. Over-the-counter probiotics and digestive aids are widely used by patients to relieve some of the leaky-gut syndrome (LGS) symptoms but there is no clinically proven treatment available.
Rutgers researchers have identified specific by-products that are released in the intestines of the host after host-microbial interaction and that protect the gut barrier. These substances have been tested in cell lines, LGS organoid and mouse models which confirmed their effectiveness in preventing barrier disruption when these models were challenged with known agents that make human guts leaky.
Market Applications:
- Supplement
- Nutraceuticals
Advantages:
- Natural products
- Safe to use
- Precise composition
Intellectual Property & Development Status: Provisional patent application filed, patent pending. Available for licensing and/or research collaboration. For any business development and other collaborative partnerships contact marketingbd@research.rutgers.edu