 Schematic of N-GLYcanyzer PAT Workflow for Integrated Upstream Analysis of Monoclonal Antibody Titer and N-linked Glycosylation
Schematic of N-GLYcanyzer PAT Workflow for Integrated Upstream Analysis of Monoclonal Antibody Titer and N-linked Glycosylation
Invention Summary:
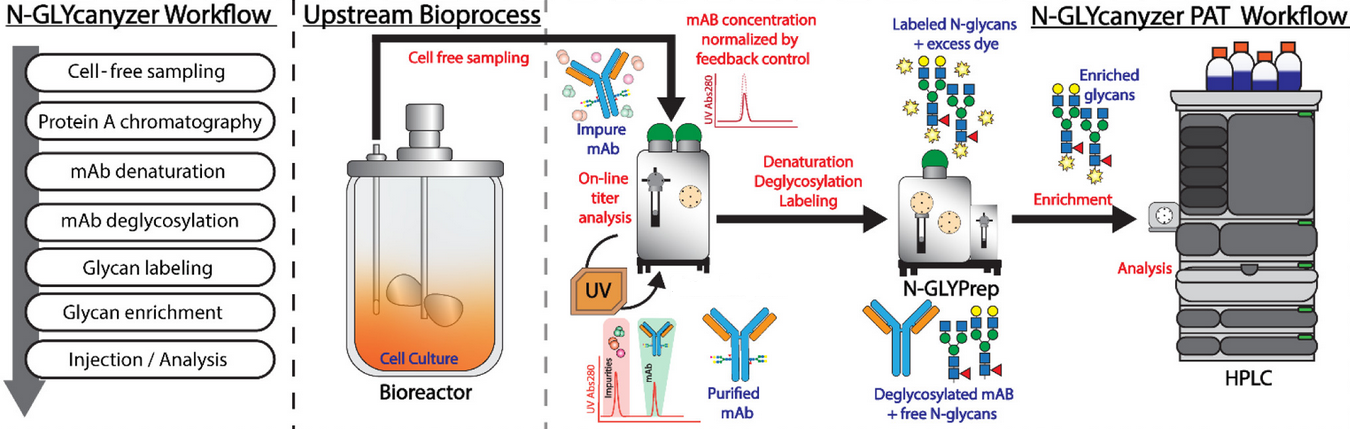
Continuous process for biologic manufacturing in biopharmaceutical industry relies on prompt feedback of process parameters for decision making as they may quickly influence the critical quality attributes (CQA’s) of the biologic that may alter drug efficacy and safety. N-linked Glycosylation is an important CQA known to influence the efficiency and safety of biologics and is known to change throughput of the upstream bioprocess. Current kit-based methods for monitoring glycosylated products, such as monoclonal antibodies (mAbs), are not suitable for real time monitoring in fully automated workflow, hence the use of process analytical technology (PAT) would enable rapid monitoring of the process to increase process knowledge and allow for tight process control.
Rutgers researchers have developed a fully automated sequential injection-based PAT system, the N-GLYcanalyzer, that can be integrated into the upstream biomanufacturing workflow to monitor glycosylation of biologics in real-time. The N-GLYcanalyzer can perform sample processing and enrichment for direct injection and analysis on an integrated high performance liquid chromatography (HPLC) system for near-real-time determination of N-glycosylation of biologics manufactured in a bioreactor.
Advantages:
- Automated on-line workflow for the biomanufacturing environment
- High efficiency in N-linked glycosylation detection
- Cost and time effective
Market Applications:
- Automated and integrated near-real-time PAT system for monitoring N-linked glycosylation of biologics during upstream biomanufacturing, as well as downstream, for real-time product release
Publications:
Intellectual Property & Development Status:
Patent pending. Available for licensing and/or search collaboration. For any business development and other collaborative partnerships contact marketingbd@research.rutgers.edu