Our major research and product development areas include: (i) probiotic-derived natural antimicrobials for human health (ii) novel probiotics for traditional and non-dairy applications. We isolated and characterized antimicrobial proteins from the known strain of Lactobacillus sporogenes (Bacillus coagulans) and from a novel strain of Bacillus amyloliquefaciens. The antimicrobial peptide subtilosin produced by B. amyloliquefaciens is active against vaginal pathogens, has spermicidal activity and kills herpes viruses 1 and 2. It also kills foodborne pathogen Listeria monocytogenes, including its mutants, resistant to a natural food preservative nisin, making it a good candidate on a role of a natural food preservative. We are working on probiotics for various applications, especially in non-dairy products. • Lactospore®: Room Temperature Stable Probiotic L. sporogenes (B. coagulans) formulation was developed by Sabinsa Corp. and marketed by this company as a commodity. In addition to increased viability in the gastrointestinal tract, its resistance to heat and other adverse conditions presents the opportunity to expand the current probiotic market to include in soft drinks, energy drinks, fruit drinks, and other non-dairy products. Lactospore® has a well established safety profile and has been granted GRAS (generally recognized as safe) status. The health benefits of probiotics in general are known to include improvement of intestinal function, bolstering of the immune system, treatment and prevention of diarrhea, restoration and maintenance of normal intestinal microflora, among others.
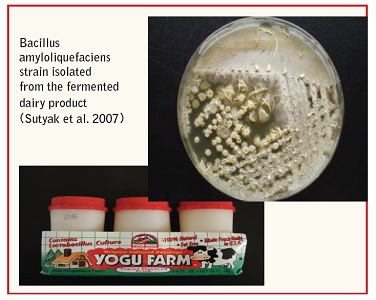
• B. amyloliquefaciens and B. subtilis Stable Probiotics B. amyloliquefaciens KATMIRA1933 was found in the fermented dairy product YoguFarm™ catalog number 74699‐02905, and was isolated from several independent batches of this product over the period of three years. These bacteria are naturally present in the product commonly consumed by humans and are therefore safe by definition. Numerous strains of B. subtilis characterized as being safe and having properties of probiotics but so far have not been sufficiently studied and not used in food products.
• Narine: a potent probiotic with several decades of documented safe applications. Lactobacillus strain 317/402 was characterized as L. acidophilus by Dr. Klaenhammer (NCSU, Raleigh, personal communication) using species‐specific probes or as L. crispatus by partial 16S rDNA analysis (Dr. Sanders, personal communication). It is used in probiotics formulations in the ex‐ Soviet Republics (Armenia, Russia, Ukraine, etc.). This strain was isolated in 1964 as a part of healthy infant microflora. The antibacterial properties of Lactobacillus strain 317/402 fermentation products have been previously demonstrated in clinical trials for systemic human disease and reportedly the strain‐based products are sold in 5 forms in the ex‐Soviet Republics for various indications such as GI and inflammatory conditions etc. Narine culture can be directly formulated into food, an orally administrable capsule or tablet, a topical ointment, or a suppository. Alternatively, the culture can be used to produce biologically active, fermented milk products, such as flavored drinks, ice creams, or yogurts. These products are not only therapeutic, but also highly nutritious. They are rich in proteins, high in essential amino acids including lysine and methionine, and have a vitamin content that is enhanced over that of plain, whole milk.
Research and Development Status: To be provided to parties interested in licensing, research collaborations, investment, etc…
Confidential Information: A confidentiality agreement is required to allow sharing of more information, including a very new and different strategy in developing probiotic-based health-promoting products.
• Stable in gastrointestinal tract and adverse environments
• Resulting product may be stored at room temperature
• Applicable for use in non-dairy products
• Established safety profile
Intestinal hea lth, immune system health, dietary supplements, cholesterol reduction, detoxification, lactose tolerance, skin allergy, among others.
Intellectual Property(Patent) Status: IP protection may be possible, with several options available for patents, know-how protection, strain deposit to the ATCC, etc.
https://rutgers.technologypublisher.com/files/sites/chikindas-probiotics.jpg
Supplementary Material
Invention Summary
Market Application
Advantages
Intellectual Property & Development Status