
Invention Summary:
Buchwald dialkylbiarylphosphines are the most widely used class of ligands in catalysis. The resulting cross-coupling reactions of this class of catalysts have a wide range of applications in both the industry and academia, especially in the fields of medical chemistry, drug discovery, biochemistry, geochemistry, natural product synthesis, small molecule synthesis, and polymer synthesis.
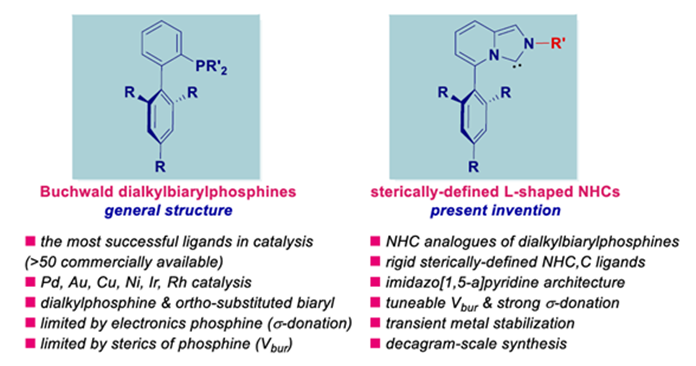
Rutgers Scientists have invented a sterically defined L-shaped NHC ligands analogous to Buchwald dialkylbiarylphosphines. This novel sterically defined L-shaped biaryl NHC ligands on imidazo[1,5-a] pyridine architecture is well defined and stable both in air and moisture complexes with transition-metals and has applications in palladium catalyzed cross-reactions. These ligands can be used to producing commercial salts and complexes at a highly scalable process using Kumada crossing coupling approach, which bypasses the use of chromatography. Many studies have been done to demonstrate the novel features of the ligands including their stable shape and their efficacy in producing salts and complexes that are commercially valued.
Market Applications:
• Ligands that can help with stable cross-coupling reactions to produce salts and complexes that are widely used in many fields.
Advantages:
- Novel Sterically- defined L-shaped.
- Robust chemical stability during the reaction in various conditions.
- Can be used to produce commercial salts and complexes used by many fields.
Intellectual Property & Development Status: Provisional patent submitted. Available for licensing and/or research collaboration.