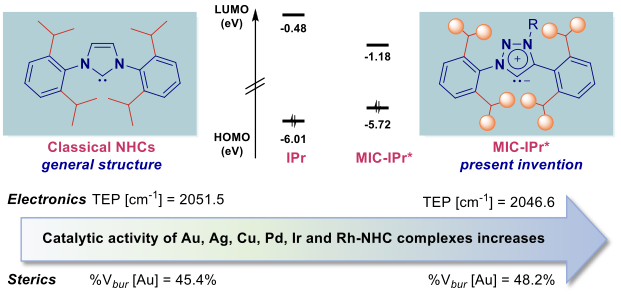
General structure of classical NHCs and the present invention
Invention Summary:
NHC ligands (NHC = N-heterocyclic carbene) are the most popular ligands in transition-metal-catalysis, organocatalysis, organic synthesis and materials science, among other applications.
Rutgers researchers have developed a novel sterically-hindered and strongly sigma-donating NHC ligands on 1H-1,2,3-triazol-5-ylidene architecture. The ligands belong to a class of abnormal N-heterocyclic carbenes, where the metal is bound to the C4 or C5 position of the heterocyclic ring rather than in the typical C2 position. This results in an overwhelmingly stronger s-donation as compared to the classical imidazol-2-ylidenes, a property that is critical for transition-metal-catalysis using a variety of metals, catalytic reactions, and mechanisms.
Market Applications:
- Pharmaceutical synthesis
- Fine chemical production
- Functional materials and polymers synthesis
- Development of catalysts for various synthetically-valuable reactions
Advantages:
- Higher stability to the metal-NHC bond and the complex itself
- Adjusts the reactivity of metals through the steric demand
- Offers stronger s-donation and p-acceptance compared to phosphine ligands
- Provides unique umbrella shape of the steric bulk around the donor center
- Facilitates peripheral N-wingtip and scaffold modification to tune steric and electronic properties of the ligand
- TEP up to 2046.8 cm-1, %Vbur up to 48.2%
- High activity in Au, Cu, Ag, Pd, Ir, Rh catalysis
Intellectual Property & Development Status: Provisional patent application filed, patent pending. Available for licensing and/or research collaboration. For any business development and other collaborative partnerships contact marketingbd@research.rutgers.edu.