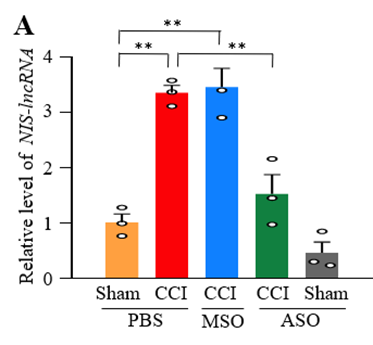
Relative level of nerve injury-specific RNAs in mice with sham surgery or chronic constriction injury (CCI) intrathecally administered PBS, missense oligonucleotides (MSO) or the herein designed antisense oligonucleotides (ASO) 7 days post-surgery.
Invention Summary:
7% to 10% of the general population endure neuropathic pain, which makes up about 20% to 25% of people suffering with chronic pain. For instance, neuropathic pain is accounted for 30% to 50% of those with spinal cord injury, 21% to 50% of Type 2 Diabetes Mellitus patients, and by 18% to 28% of cancer patients. Under certain conditions, the etiology of neuropathic pain stems from a dysregulation of pain–associated genes located within the dorsal root ganglia (DRG).
Rutgers researchers have identified a nerve injury-specific long noncoding RNA (NIS-IncRNA) that is upregulated in DRG neurons following nerve trauma, in cases of diabetes, or following chemotherapeutic exposure. Genetic knockdown of NIS‐lncRNA in injured DRG through DRG microinjection of specific NIS‐lncRNA siRNA in CD1 mice or AAV5‐Cre in NIS‐lncRNA floxed mice attenuated neuropathic pain. Consequently, the inventors designed antisense oligonucleotides (ASO) that knock down the long noncoding RNA without inducing toxicity, carrying the potential to treat neuropathic pain.
Market Applications:
- Neuropathic pain caused by nerve trauma.
- Neuropathic pain in diabetes.
- Neuropathic pain treatment following chemotherapy.
Advantages:
- Less addictive than opioid analgesics typically administered for neuropathic pain.
- Does not affect basal/acute pain and locomotor function.
- Injectable intrathecally or subcutaneously.
- Single administration produces long-lasting antinociceptive effects on neuropathic pain.
Publications:
- Du S., et al. (2022) A nerve injury-specific long noncoding RNA promotes neuropathic pain by increasing Ccl2 expression. JCI, 132; e153563.
- Wen CH et al., (2023) Effect of intrathecal NIS-lncRNA antisense oligonucleotides on neuropathic pain caused by nerve trauma, chemotherapy, or diabetics. BJA, 130; 202-216
Intellectual Property & Development Status: Provisional filed. Patent pending. Available for licensing and/or search collaboration. For any business development and other collaborative partnerships contact: marketingbd@research.rutgers.edu