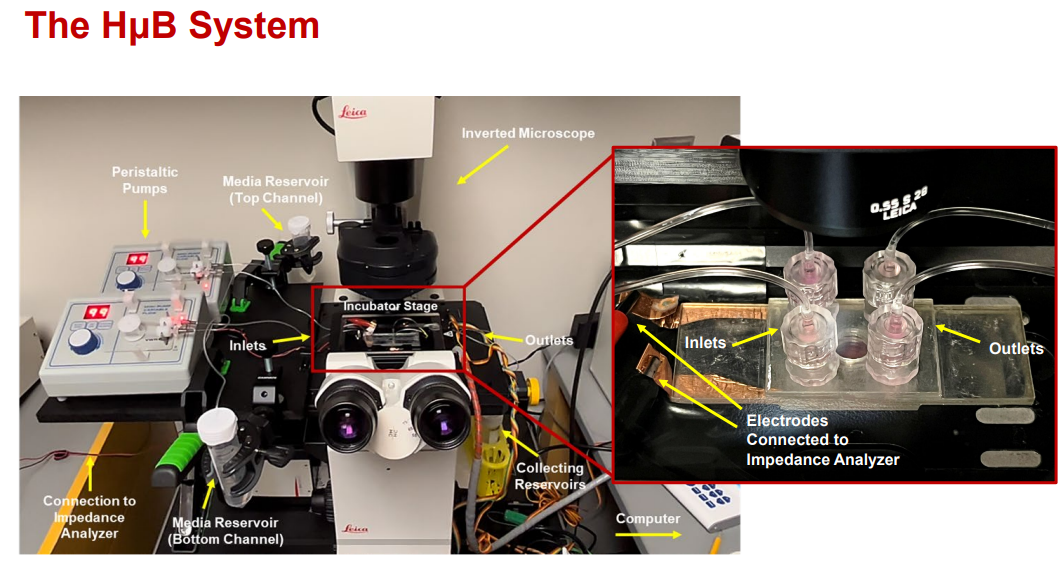
Figure 1. The Hμb being used with a conventional microscope stage.

Invention Summary:
In the pharmaceutical and cosmetics industries, it is pertinent to screen for safety and dosage efficacy of developed drugs and compounds. These experiments can be costly and use equipment that can only be operated by skilled technicians and experts.
Rutgers researchers have developed a novel microfluidic device able to examine the integrity of cell barriers, such as vascular barriers along different parts of the brain, spinal cord, gut barrier, pulmonary barrier, and the blood retinal barrier. The platform facilitates the measurement of resistivity and permeability across individual and/or combined monolayers under physiological flow conditions over time. Changes in cell response to natural bio‐compounds (such as glucose) as well as commercial pharmacology (such as anti-angiogenic drugs) can be measured with high sensitivity.
The device is manufactured by laser cutting acrylic sheets removing the need for cleanroom facilities. The Hμb features plastic clear electrodes, which permit brightfield/fluorescence imaging while recording barrier resistivity in real time. Likewise, this device incorporates 2 independent flow rates along the cell barrier resulting in a dynamic and customizable system.
Market Applications:
- R&D testing platform
- Bridge between in vitro and in vivo models
- Drug screening tool
Advantages:
- Low-cost manufacturing
- Quick assembly time
- Easy-to-use
- Variable flow rates
- Long runtime capability
- Interstitial and capillary flowrate measurement capabilities
Intellectual Property & Development Status: Provisional patent application filed, patent pending. Available for licensing and/or search collaboration. For any business development and other collaborative partnerships contact marketingbd@research.rutgers.edu