
Invention Summary:
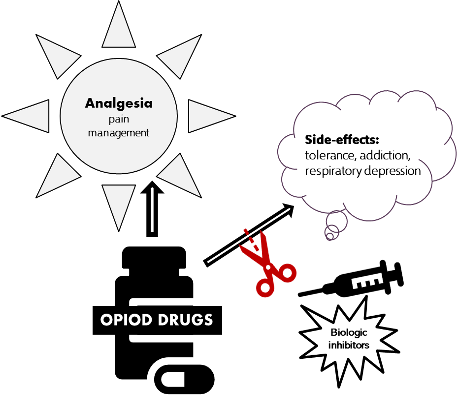
Mu opioids, including morphine, fentanyl, and oxycodone, remain the mainstream drugs for moderate-to-severe pain management in the clinic despite their side-effects, such as tolerance, reward, addiction, and respiratory depression. However, misusing these mu opioids can magnify their side-effects and promote the development of opioid use disorder, a main cause of the worldwide opioid epidemic and opioid overdose deaths, which have been climbing steeply in the United States in recent years. There is a dire need for developing novel therapeutic strategies and medications that can be potent in analgesia for different pain conditions but have no adverse side-effects associated with traditional mu opioids.
Rutgers researchers have identified the specific variant of a highly conserved mu-opioid receptor gene, OPRM1, that mediates several adverse effects associated with the clinical use of opioids. The team has also developed biologic inhibitors (nanobody and/or antisense oligonucleotide) targeting the specific variant. These inhibitors can be strategically co-administered with the opioids to mitigate the adverse side effects without altering analgesia. Observations have been confirmed in mouse models and studies in monkey models are underway.
Market Applications:
- Therapeutic, co-administered with mu-opioids during pain management.
- Preventive for side effects of opioid drug use.
Advantages:
- Mitigate adverse side-effects of clinically used opioids.
- Reduce opioid tolerance and abuse
Intellectual Property & Development Status: Provisional patent application filed, patent pending. Available for licensing and/or search collaboration. For any business development and other collaborative partnerships contact marketingbd@research.rutgers.edu