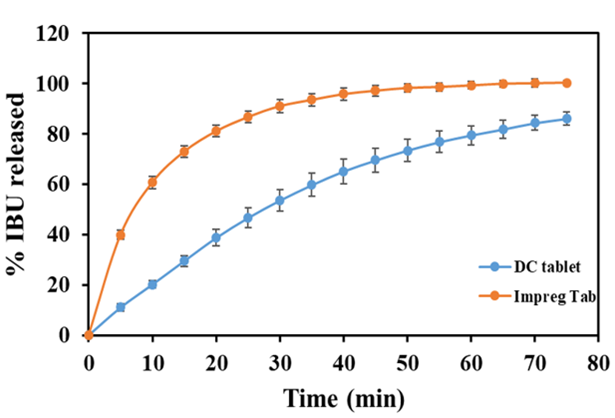

A comparison of dissolution profiles for impregnated ibuprofen (IBU) tablets and direct compression IBU tablets
|
Invention Summary:
A major challenge in pharmaceutical product development is the formation of homogenous blends containing small amount of active pharmaceutical ingredients (APIs). Poor mechanical properties of APIs further exacerbate the problem.
A Rutgers research team led by Prof. Muzzio has developed and tested a new pharmaceutical product development process by combining continuous manufacturing with API impregnation. Impregnation of APIs into porous carriers eliminate the influence of the undesired physical properties of the APIs and continuous manufacturing facilitates the achievement of homogeneity of APIs in the final products. Specifically, the research team has demonstrated the feasibility of using a continuous horizontal tubular blender to implement continuous impregnation and the use of a continuous process to manufacture drug products composed of API-impregnate carriers.
Market Applications:
• A platform technology for API formulation combining impregnation and continuous manufacturing
Advantages:
- Simple & scalable
- Capable of achieving uniform products containing 0.1% to 10% APIs
- Elimination of the use of expensive excipients
- Capable of developing a generic platform for product development that suits many different types of APIs
Intellectual Property & Development Status: Patent pending. Available for licensing and/or research collaboration.