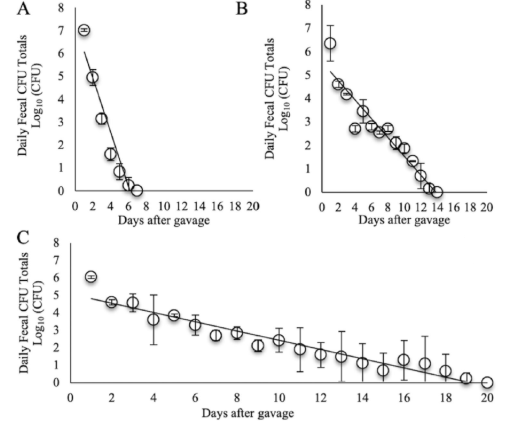

Recovered CFUs from daily fecal matter after repetitive autologous murine gastrointestinal track gavages. (A) Log10 of the CFU counts from fecal matter obtained after gavage of wild type L. rhamnosus (n=2). (B) Log10 of the CFU counts from fecal matter obtained after gavage of Evolution 1, which used colonies selected from gavage 1 (n=2). (C) Log10 of the CFU counts from fecal matter obtained after the gavage of Evolution 2 (n=2).
Invention Summary:
Lacticaseibacillus is a well‐known genus of probiotic bacteria with Lacticaseibacillus rhamnosus GG being used in clinical applications for treating diarrhea, obesity, and irritable bowel syndrome. A major limitation, however, is the low survival and persistence of these probiotics in the gut, which can reduce treatment efficacy.
This invention describes novel clones of Lacticaseibacillus rhamnosus strains with significantly improved persistence in the digestive tract. Through a method of a series of autologous gavages in mice, bacterial isolates with nearly triple the gut retention time compared to the original strain were selected. These evolved isolates show increased resistance to bile salts and possess multiple genetic changes that promote probiotic survival, although with reduced adhesion to intestinal cells.
Market Applications:
- Development of next-generation probiotics with extended digestive tract colonization.
Advantages:
Publications:
Intellectual Property & Development Status: Provisional application filed. Patent pending. Available for licensing and/or research collaboration. For any business development and other collaborative partnerships, contact: marketingbd@research.rutgers.edu