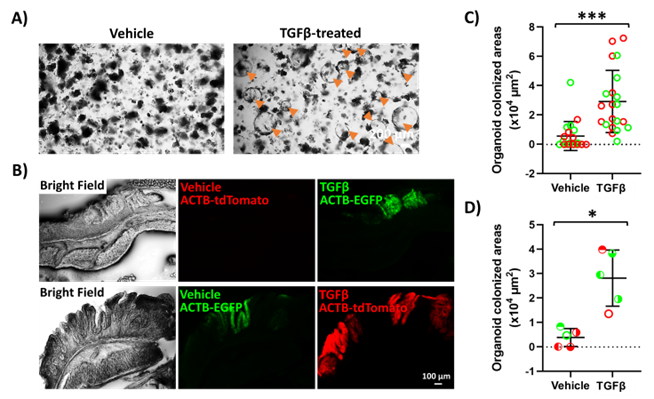
A) GI epithelial organoids treated with TGFβ (right panel) acquire a spherical morphology and regenerative identity compared to vehicle-treated organoids (left panel). B-D) Organoids treated with TGFβ engraft more efficiently into the colon of mice exhibiting ulcerative colitis-like disease based on fluorescent micrographs (B), size per colonized area (C) and average size of colonized area per mouse (D).
Invention Summary:
The gastrointestinal (GI) tract consists of epithelial cells that are essential for tissue function but susceptible to damage and the functional cell of some congenital diseases. Damage to the GI tract can arise from cancer therapies, like radiotherapy and chemotherapy, as well as inflammatory diseases. To improve the symptoms of GI tract injury, tissue transplantation/grafting is often considered, and the first clinical trials for organoid transplants began last year. GI transplants are expected to be a major clinical advance in the coming years, however, there is a need to improve the efficiency of transplantation in order to bring organoid transplant technology more rapidly and efficiently to patients.
Rutgers researchers have developed a novel method for improving the transplantation of epithelial cells to the damaged GI tract. They found that treating organoids with transforming growth factor beta (TGFβ) increased expression of genes associated with regeneration and stemness (see preprint). The researchers further show that the cells treated with TGFβ have improved grafting capacity in mouse models of damaged GI tract. These findings offer a potential method for improving grafting capacity of organoids, specifically in the context of GI epithelia. These advances could have broad applications in the field of regenerative medicine, with applications for enhancing engraftment in specific genetic deficiencies and treatment of ulcerated epithelia like Inflammatory Bowel Disease. This technology also has the potential to be applied for engraftment of other tissues across diverse mucosal barriers.
Market Applications:
- Tissue/organ transplantation/engraftment
- 3D cell culture research
- Medical devices
Advantages:
- Treatment of cells prior to tissue transplantation to avoid in vivo side effects
- Cost-effective
- Broadly applicable to mucosal tissues
Intellectual Property & Development Status: PCT application filed, patent pending. Available for licensing and/or research collaboration. Please contact marketingbd@research.rutgers.edu.