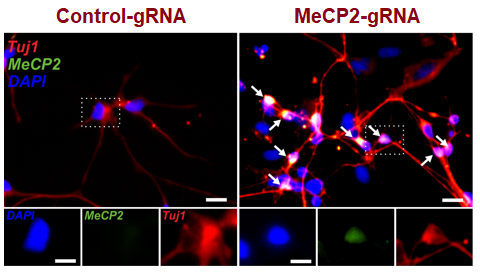
Successful gene editing in neurons differentiated from patient-derived neural stem cells. Left panel, gene editing using control guide RNA; Right panel, gene editing using MeCP2 guide RNA.
|
Invention Summary:
CRISPR-Cas-mediated genome editing makes effective gene therapy of human disease feasible. Although viral delivery of CRISPR-Cas agent is quite efficient, the safety concern limited its clinic applications. On the other hand, the current available non-viral delivery methods can’t reach the goal for effective genome-editing of cells for clinic application.
Rutgers Scientists have developed a novel non-viral CRISPR-Cas9 gene editing platform - MAGE (Magnetic nanoparticle Assisted Genome Editing) which utilizes a specifically designed delivery system for introducing gene editing materials into cells. The team has tested the efficiency of this platform in correcting a mutation in the MeCP2 gene which is known to be associated with the genetic disorder Rett syndrome. Successful gene editing has been demonstrated in the mutated MeCP2 locus in iPSC-derived neural progenitor cells obtained from a Rett Syndrome patient. This process is highly efficient with 43% of cells that have received the CRISPR/Cas9 gene editing system showing correction of the MeCP2 mutation. Furthermore, neurons derived from these gene-edited cells show functional recovery of neural activities.
Advantages:
- Non-viral system
- One-shot delivery for all gene editing materials
- High gene editing efficiency
Market Applications:
Delivery of genome-editing agent for
- Gene therapy of human diseases
- Generating gene-edited cell lines
Intellectual Property & Development Status: Patent Pending. Available for licensing and/or research collaboration.