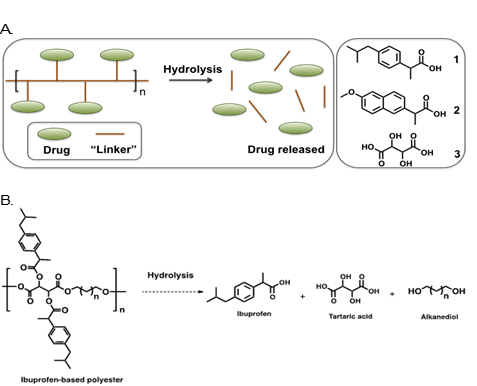
A. Drug delivery system hydrolysis – composed of 1 (Ibuprofen), 2 (naproxen) and 3 (tartatic acid) into polymer backbones. B. Hydrolytic degradation of the ibuprofen-based polyester.
Invention Summary:
Ibuprofen and naproxen are commonly used to treat pain and swelling associated with rheumatoid arthritis and osteoarthritis. These non-steroidal anti-inflammatory drugs (NSAIDs) have relatively short half-lives upon ingestion. When orally administered, NSAIDs may cause severe gastrointestinal side effects such as stomach ulceration, bleeding, and perforation. Drug delivery systems have been developed to localize the drug, thereby decreasing the side effects associated with systemic drug administration and increasing the duration of the drug effect. However, these systems typically have low NSAID loadings and burst release issues.
Rutgers researchers have developed a drug delivery system to localize NSAID release and prolong it’s effects. In this technology, the NSAID is chemically incorporated into biodegradable polymer backbones creating a unique drug delivery platform. The system comprises of ibuprofen and naproxen incorporated into synthetic polyesters with tartaric acid (an antioxidant) and alkanediols. The polyesters are biodegradable due to the labile ester bonds, which upon hydrolytic degradation releases the anti-inflammatory drug, along with antioxidants to further mitigate inflammation and pain. The polymer system shows more controlled and tunable prolonged release profile and with no burst release of NSAIDs.
Applications
- In addition to oral delivery platforms, polymers can be formulated into implantable and injectable delivery systems for localized inflammation treatment
- NSAID inflammatory drug release activity testing
Advantages
- Novel, biodegradable delivery systems with potentially higher NSAID loadings and more controlled release characteristics than current systems
- Polymers exhibit significantly higher NSAID loadings (~65-75%)
- Polymers have potential for greater range of release rates and formulations than current NSAID platforms.
Intellectual Property & Development Status: Patent granted US9144579B2. Available for licensing and/or research collaboration.