Modified Kisspeptin Receptor Agonist as Novel Treatment of MASLD
Web Published:
10/22/2020
Invention Summary:
Metabolically Dysfunctional-Associated Steatotic Liver Disease (MASLD), previously known as non-alcoholic fatty liver disease (NAFLD), is a highly prevalent chronic liver disease, affecting 38% of all adults and up to 14% of children, globally. It is anticipated that by 2040, the MASLD prevalence rate will increase to 55%, worldwide. In the U.S, nearly 86.3 million adults currently have MASLD. MASLD initiates with steatosis (lipid accumulation in the liver), which can then progress to steatohepatitis, fibrosis, cirrhosis and eventual liver failure. MASLD is now a leading cause of liver transplantation. Existing experimental treatments often target late-stage disease or address metabolic factors indirectly, but none have demonstrated rapid, direct reversal of liver pathology. A therapy that can both halt disease progression and actively reverse fibrosis would represent a breakthrough in the field.
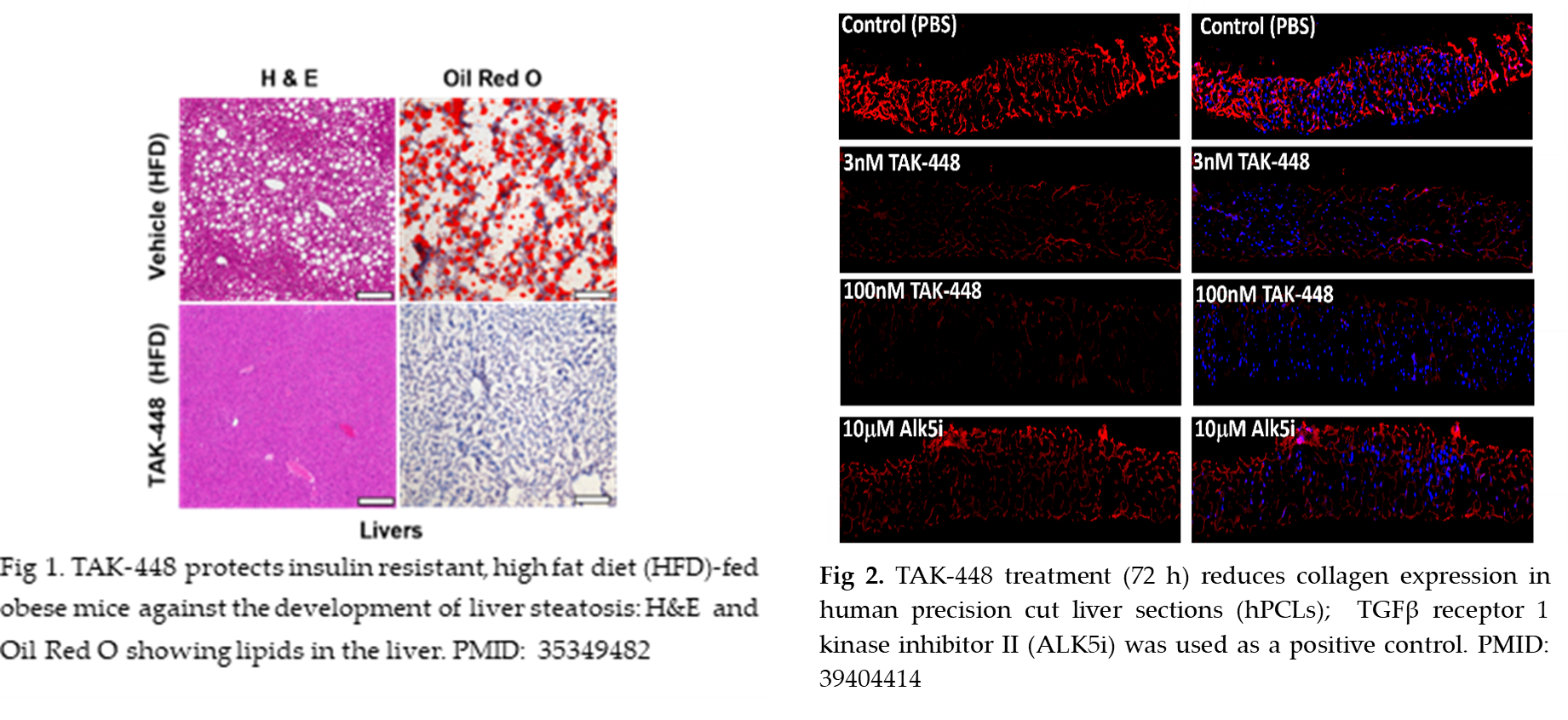
Rutgers researchers have developed a novel treatment for targeting all stages of MAFLD (steatosis, steatohepatitis, and fibrosis), namely the modified kisspeptin analog, TAK-448 (MVT-602), originally developed for endocrine disorders. Published studies using several mouse models of MASLD demonstrate that TAK-448 treatment protects against steatosis by inhibiting the synthesis of new fatty acids (de novo lipogenesis), thereby reducing steatosis, liver triglycerides and metabolic dysfunction. TAK-448 treatment improves insulin sensitivity, lowers inflammatory and fibrotic markers, and decreases liver fibrosis. Most strikingly, in human liver slices (hPCLs) from patients with Stage 1, 2, or 4 fibrosis, TAK-448 reversed fibrosis within 72 hours — an unprecedented finding in this disease space. TAK-448 treatment reduced collagen secretion and lowered the expression of markers of fibrogenesis (collagen, smooth muscle actin, fibronectin) and inflammatory markers (interleukin-6, TNFa). Unlike current experimental approaches that primarily slow progression, TAK-448 offers a disease-modifying strategy that intervenes at multiple points in disease biology, combining metabolic improvement, anti-inflammatory action, and rapid fibrosis reversal into a single therapeutic platform.
Market Applications:
Advantages:
Publications:
- Guzman S, Dragan M, Kwon H, de Oliveira V, Rao S, Bhatt V, Kalemba KM, Shah A, Rustgi VK, Wang H, Bech PR, Abbara A, Izzi-Engbeaya C, Manousou P, Guo JY, Guo GL, Radovick S, Dhillo WS, Wondisford FE, Babwah AV, Bhattacharya M. Targeting hepatic kisspeptin receptor ameliorates nonalcoholic fatty liver disease in a mouse model. J Clin Invest. 2022 May 16;132(10):e145889. doi: 10.1172/JCI145889. PMID: 35349482; PMCID: PMC9106350.
- Prasad K, Bhattacharya D, Shams SGE, Izarraras K, Hart T, Mayfield B, Blaszczyk MB, Zhou Z, Pajvani UB, Friedman SL, Bhattacharya M. Kisspeptin Alleviates Human Hepatic Fibrogenesis by Inhibiting TGFβ Signaling in Hepatic Stellate Cells. Cells. 2024 Oct 4;13(19):1651. doi: 10.3390/cells13191651. PMID: 39404414; PMCID: PMC11476267.
Intellectual Property & Development Status: PCT application filed. Patent pending. Available for licensing and/or research collaboration. For any business development and other collaborative partnerships, contact: marketingbd@research.rutgers.edu
Patent Information:
| Title |
App Type |
Country |
Serial No. |
Patent No. |
File Date |
Issued Date |
Expire Date |
Patent Status |
|
|
|
ID: 2020-071
Category:
Inventors:
Keywords:
|